NEW! The Gist (FREE) | E-BOOKS |
(IGP) GS Paper 1 - General Science - "Gist of Physics from NCERT Books"
Integrated Guidance Programme of General Studies for IAS (Pre) - 2013
Subject - General Science
Chapter : Gist of Physics from NCERT Books
Atomic Physics:
- An atom is the smallest particle of the element that can exist independently and retain all its chemical properties.
- Dalton’s atomic theory, which suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory.
Thomson proposed that:
- An atom consists of a positively charged sphere and the electrons are embedded in it.
- The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
- Rutherford’s alpha-particle scattering experiment led to the discovery of the atomic nucleus. Rutherford’s model of the atom proposed that a very tiny nucleus is present inside the atom and electrons revolve around this nucleus. The stability of the atom could not be explained by this model.
- Neils Bohr’s model of the atom was more successful. He proposed that electrons are distributed in different shells with discrete energy around the nucleus. If the atomic shells are complete, then the atom will be stable and less reactive.
- J. Chadwick discovered presence of neutrons in the nucleus of an atom. So, the three sub-atomic particles of an atom are: (i) electrons, (ii) protons and (iii) neutrons. Electrons are negatively charged, protons are positively charged and neutrons have no charges. The mass of an electron is about 1/2000 times the mass of an hydrogen atom. The mass of a proton and a neutron is taken as one unit each.
Isotopes:
- Isotopes are atoms of the same element, which have different mass numbers.
Isobars:
- Isobars are atoms having the same mass number but different atomic numbers.
Radioactivity:
-
Radioactivity occurs when an atomic nucleus breaks down into smaller particles. There are three types of nuclear radiation: alpha, beta, and gamma. Alpha particles are positively charged, beta particles are negatively charged, and gamma particles have no charge. The radiations also have increasing levels of energy, first Alpha, then Beta, and finally Gamma, which is the most energetic of all these. Alpha and Beta are particles, but Gamma is a wave.
- When a radioactive nucleus changes, the remaining nucleus (and atom) is not the same as it was. It changes its identity.
For Detail Description, Analysis and More MCQs of the Chapter Buy this Study Notes:
Fission:
- Fission is the splitting of an atom. Not all atoms will go through fission; as a matter of fact, very few do under normal circumstances.
Fusion:
- Fusion is the process of two small atomic nuclei coming together to make a larger nucleus which is stable. The simplest nuclei to use are deuterium and tritium (isotopes of hydrogen).
Heat:
- Temperature is a relative measure, or indication of hotness or coldness.
- Heat is the form of energy transferred between two (or more) systems or a system and its surroundings by virtue of temperature difference. The SI unit of heat energy transferred is expressed in joule (J) while SI unit of temperature is kelvin (K), and °C is a commonly used unit of temperature.
- Thermometer is a device used for measuring temperatures. The two familiar temperature scales are the Fahrenheit temperature scale and the Celsius temperature scale. The Celsius temperature (tC) and the Farenheit temperare (tF) are related by: tF = (9/5) tC + 32
- In principle, there is no upper limit to temperature but there is a definite lower limit- the absolute zero. This limiting temperature is 273.16° below zero on the celsius scale of temperature.
Light:
LIGHT TRAVELS ALONG A STRAIGHT LINE
- Light is reflected from all surfaces. Regular reflection takes place when light is incident on smooth, polished and regular surfaces.
-
After striking the surface, the ray of light is reflected in another direction. The light ray, which strikes any surface,is called the incident ray. The ray that comes back from the surface after reflection is known as the reflected ray.
- The angle between the normal and incident ray is called the angle of incidence . The angle between the normal and the reflected ray is known as the angle of reflection.
- Two laws of reflection are:
- The angle of incidence is equal to the angle of reflection.
- Incident ray, reflected ray and the normal drawn at the point of incidence to the reflecting surface, lie in the same plane.
Lens:
- Lenses are widely used in spectacles, telescopes and microscopes. Those lenses which feel thicker in the middle than at the edges are convex lenses. Those which feel thinner in the middle than at the edges are concave lenses. Notice that the lenses are transparent and light can pass through them.
Convex Lens:
- A convex lens converges (bends inward) the light generally falling on it. Therefore, it is called a converging lens. On the other hand, a concave lens diverges (bends outward) the light and is called a diverging lens.
Concave Lens:
- A concave lens always forms erect, virtual and smaller image than the object.
Total Internal Reflection:
-
Total internal reflection is the phenomenon which involves the reflection of all the incident light off the boundary. Total internal reflection only takes place when both of the following two conditions are met: (i) the light is in the more dense medium and approaching the less dense medium., and (ii) the angle of incidence is greater than the so-called critical angle. Total internal reflection will not take place unless the incident light is traveling within the more optically dense medium towards the less optically dense medium.
Dispersion of Light:
- It is the phenomenon of splitting of a beam of white light into its constituent colors on passing through prism. The order of colors from the lower end are violet, indigo, blue, green, yellow, orange and red. At one end of the band, there is red and at the other violet. The sequence of colours can be best remembered by the wordVIBGYOR’ which is formed by taking the initial letter of each colour.
- A laser is just a really powerful beam of light. Laser isn’t a word but an acronym. It stands for LIGHT AMPLIFICATION by STIMULATED EMISSION of RADIATION.
Magnetism and Electricity:
A. Magnetism
The word magnet is derived from the name of an island in Greece calledMagnesia where magnetic ore deposits were found, as early as 600 BC. Magnetite, an iron ore, is a natural magnet. It is called lodstone.
The properties of a magnet are
- it attracts small piece of iron towards it.
- it always cmes to rest in north-south direction when suspended freely.
- like poles repel, unlike poles attracts each other
- Magnetic poles always exist in pairs.
- the strength of a magnet is maximum at poles located near the poends
B. Electricity
- The phenomenon due to which a suitable combination of bodies on rubbing, get electrified is called electricity. If a charge on a body is not allowed to flow, it is called the static electricity.
- There are two different types of electric charges namely the positive and negative charges. Like charges repel and unlike charges attract each other.
-
Electric current always flows from the point of high potential. The potential difference between two conductors is equal to the work done in conducting a unit positive charges from one conductor to the other conductor through a metalic wire.
-
The flow of charge is called the current and it is the rate at which electric charges pass though a conductor. The charged particle can be either positive or negative. In order for a charge to flow, it needs a push (a force) and it is supplied by voltage, or potential difference. The charge flows from high potential energy to low potential energy.
Electromagnetism:
- The branch of physics which deals with the relationship between electricity and magnetism is called electomagnetism.
- Faraday’s law of induction is one of the important concepts of electricity. It looks at the way changing magnetic fields can cause current to flow in wires. Basically, it is a formula/concept that describes how potential difference (voltage difference) is created and how much is created. It’s a huge concept to understand that the changing of a magnetic field can create voltage.
Coulomb’s Law:
- Coulomb’s Law is one of the basic ideas of electricity in physics. The law looks at the forces created between two charged objects. As distance increases, the forces and electric fields decrease. This simple idea was converted into a relatively simple formula. The force between the objects can be positive or negative depending on whether the objects are attracted to each other or repelled.
Mechanics:
- Motion: In physics, motion is change of location or position of an object with respect to time. Mechanical motion is of two types, transitional ( linear ) and rotational ( spin).
- SPEED: The speed of a moving body is the rate at which it covers distance i.e. the distance it covers per unit of time.
- VELOCITY: The distance covered by an object in a specified direction in unit time interval is called velocity. The S.I. Unit of velocity is m/s.
- ACCELERATION: When an object’s velocity changes, it accelerates. Acceleration shows the change in velocity in a unit time. Velocity is measured in meters per second, m/s, so acceleration is measured in (m/s)/s, or m/s2, which can be both positive and negative. The symbol for acceleration is a (boldface).
Newton’s Laws of Motion:
1. Newton’s First Law of Motion:
-
Newton’s first law of motion states that “An object at rest tends to stay at rest and an object in motion tends to stay in motion with the same speed and in the same direction unless acted upon by an unbalanced force.” . Every object in a state of uniform motion tends to remain in that state of motion unless an external force is applied to it.
2. Newton’s Second Law of Motion:
- The acceleration of an object as produced by a net force is directly proportional to the magnitude of the net force, in the same direction as the net force, and inversely proportional to the mass of the object.
3. Newton’s Third Law of Motion:
- For every action, there is an equal and opposite reaction.
-
The statement means that in every interaction, there is a pair of forces acting on the two interacting objects. The size of the forces on the first object equals the size of the force on the second object. The direction of the force on the first object is opposite to the direction of the force on the second object. Forces always come in pairs - equal and opposite action-reaction force pairs.
Properties of Matters:
-
A matter can neither be created nor it can be destroyed but it can be transformed from one state to another. Matter is made of basic building blocks commonly called elements which are 112 in number. The matter is made of only one kind of element then the smallest unit of that element is called an atom. If the matter is made of two or more different elements then the smallest unit of matter is called a molecule.
- Molecule is defined as the smallest unit of matter which has independent existence and can retain complete physical and chemical properties of matters.
According to kinetic theory of matter:
- molecules are in the state of continuous motion in all possible directions and hence they posses kinetic energy which increases with the gain of heat energy or rise in temperature,
- the molecules always attract each other,
- the force of attraction between the molecules decreases with the increase in intermolecular spaces
Sound:
- Sound is a form of energy and like all other energies, sound is not visible to us. It produces a sensation of hearing when it reaches our ears. Sound can not travel through vacuum.
-
Sound is produced due to vibration of different objects.The matter or substance through which sound is transmitted is called a medium. It can be solid, liquid or gas. Sound moves through a medium from the point of generation to the listener.
- sound waves are longitudinal waves. Sound travels as successive compressions and rarefactions in the medium. In sound propagation, it is the energy of the sound that travels and not the particles of the medium.
-
The audible range of sound for human beings extends from about 20 Hz to 20000 Hz (one Hz = one cycle/s). Children under the age of five and some animals, such as dogs can hear up to 25 kHz (1 kHz = 1000 Hz).
- Sounds of frequencies below 20 Hz are called infrasonic sound or infrasound.
- Frequencies higher than 20 kHz are called ultrasonic sound or ultrasound. Ultrasound is produced by dolphins, bats and porpoises.
Units and Measurement:
-
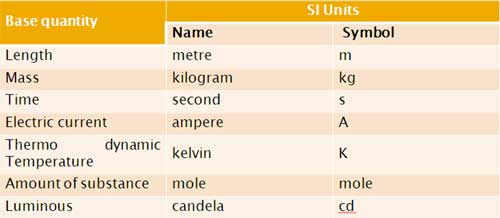
Each base quantity is defined in terms of a certain basic, arbitrarily chosen but properly standardised reference standard called unit (such as metre, kilogram, second, ampere, kelvin, mole and candela). The units for the fundamental or base quantities are called fundamental or base units.
-
Other physical quantities, derived from the base quantities, can be expressed as a combination of the base units and are called derived units. A complete set of units, both fundamental and derived, is called a system of units.
-
The SI units have well defined and internationally accepted unit symbols (such as m for metre, kg for kilogram, s for second, A for ampere, N for newton etc.). Physical measurements are usually expressed for small and large quantities in scientific notation, with powers of 10. Scientific notation and the prefixes are used to simplify measurement notation and numerical computation, giving indication to the precision of the numbers.
SI Base Quantities and Units:
Archimedes’ Principle:
- When a body is immersed fully or partially in a fluid, it experiences an upward force that is equal to the weight of the fluid displaced by it.
-
Archimedes’ principle has many applications. It is used in designing ships and submarines. Lactometers, which are used to determine the purity of a sample of milk and hydrometers used for determining density of liquids, are based on this principle.
- Density and Relative Density: The mass per unit volume of a substance is called its density. The SI unit of density is kilogram per meter cubed. Density= mass/volume.
- The relative density of a substance is the ratio of its density to that of water: Relative density = Density of a substance/Density of water. Since the relative density is a ratio of similar.
For Detail Description, Analysis and More MCQs of the Chapter Buy this Study Notes:

