(HOT) UPSC Current Affairs 2025 PDF
NEW! The Gist (NOV-2025) | E-BOOKS
UPSC Geo-Scientist and Geologist Exam Papers 2018 : Chemistry Paper - I

(Download) UPSC: Geologist Examination Papers-2018
Exam Name : UPSC Geo-Scientist and Geologist Exam 2018
Subject : Chemistry Paper - I
Year : 2018
CHEMISTRY
Paper I
ITime Allowed : Three Hours
Maximum Marks : 200
QUESTION PAPER SPECIFIC INSTRUCTIONS
Please read each of the following instructions carefully before attempting questions.
There are ELEVEN questions divided under SIX Sections.
Candidate has to attempt SIX questions in all.
The ONLY question in Section A is compulsory.
Out of the remaining TEN questions, FIVE questions are to be attempted choosing ONE from each Section.
The number of marks carried by a question / part is indicated against it.
Attempts of questions shall be counted in sequential order. Unless struck off, attempt of a question shall be counted even if attempted partly.
Any page or portion of the page left blank in the answer-book must be clearly struck off. Answers must be written in ENGLISH only.
Neat sketches may be drawn to illustrate answers, wherever required.
Unless otherwise mentioned, symbols and notations have their usual standard meanings. Assume suitable data, if necessary and indicate the same clearly.
SECTION 'A'
1. Answer all of the following:
1. (a) How lithium hydride can be prepared ? Write the product of the
reaction between lithium hydride and carbon dioxide. Whether it (LiH) is
electrovalent or covalent
compound ? Explain.
1. (b) Write IUPAC nomenclature of the following coordination compounds :
(i) [CoCl(CN)(NO2) (NH3)3]
(ii) [Pt(Py)4](PtCl4]
1. (c) A complex with the formula CoCl2-4NH; gives precipitate with AgNO3 with reference to one chloride ion. Answer the following:
(i) What is the primary valency of cobalt ?
(ii) What is the secondary valency of cobalt ?
(iii) Draw the possible geometrical isomers of the compound.
1. (d) All Bronsted-Lowry acids are Lewis acids also. But all Lewis
acids are not Bronsted-Lowry acids. Explain.
1.(e) Iodine and bromine are added to a solution containing I and Br
ions. What reaction would occur ? Explain.
The electrode potentials for the reactions are :
I2 + 2e- → 2I-, E° = +0.54 V
Br2 + 2e- → 2Br-, E° = +1.08 V
1. (f) What is 18-electron rule ? Using the 18-electron rule predict the 'n' value for the following compounds :
(i) [h6 (C6H6)
Cr(CO)n]
(ii) [h4 (C4H4)2
Mn(Cl)(CO)n]
1. (g) A wooden article of old age shows 40% as much 14C activity as a fresh piece of wood. Calculate the age of the article.
(t1/2 of 14C = 5760 years)
1. (h) What is the usual order of preference of the alkali metal ions
for a cation exchanging resin ? Explain.
1. (i) Draw the structure of borazine. With the help of its reaction with
HCl, explain why chemical properties of borazine are quite different from those
of benzene.
1. (j) Atomic radii of two of the lanthanides are considerably higher
than the other lanthanides. Identify those two lanthanides and explain the
observation.
SECTION ‘B'
(Attempt any one question)
2. (a) Define the electron affinity. Explain the general trends of
electron affinity in periodic table. Electron affinities of beryllium,
magnesium, calcium and nitrogen are practically close to zero. Explain.
2. (b) Explain the basicities of the following acids and justify the
answer by drawing the structures :
(i) Orthophosphoric acid
(ii) Pyrophosphoric acid
(iii) Metaphosphoric acid
2. (c) Write the product(s) formed from the following reactions :
(i) 3PC13 + S03 →
(ii) HPO4 + H20 →
(iii) TeO2 + 2SeF4 →
(iv) 8HI + H2SO4 →
(v) PC15 + H20 →
3. (a) Write the important points of VSEPR theory. Draw the shape of the following molecules by using VSEPR theory :
(i) SF4
(ii) XeOF4
(iii) IF5
3. (b) Draw the Born-Haber Cycle for the formation of NaCl crystal and
write the two important uses of the Cycle.
3. (c) Draw the molecular orbital energy level diagrams for nitric oxide
and carbon monoxide. Explain the magnetic behaviour of the molecules also.
SECTION ‘C
(Attempt any one question)
4. (a) Calculate ground state terms for the following electronic configurations :
(i) d3
(ii) d4
(iii) d8
(iv) f3 and
(v) f7
4. (b) Arrange the following metal compounds in the increasing order of their molar extinction coefficient values. Explain the reasons.
KMnO4, [Co(NH3)6,]2+, [MnBr4]2- and [CoC14]2-
4. (c) Calculate the crystal field stabilization energy value for the complex [Cr(NH3)6]2+. The crystal field splitting energy and pairing energy are found to be 150 k.J mol-1 and 120 k.J mol-1 respectively.
5. (a) What is Jahn-Teller effect? Calculate Jahn-Teller stabilization
energy for Cu(II) complex in an octahedral field.
5. (b) What is premature end point? Explain this with reference to
ferrous and dichromate titration in acidic medium.
5. (c) Draw the basicity order of CH-3, NH-2,
OH- and F- anions. Justify your answer.
SECTION ‘D'
(Attempt any one question)
6. (a) Balance the following equation by ion-electron method in basic medium :
P4 → H2PO2- + PH3
6. (b) Distinguish between disproportionation and comproportionation reactions. Classify the following reactions into disproportionation or comproportionation reactions. Explain the reasons.
(i) 3Cl2 + 6NaOH → NaClO3 + 5NaCl + 3H20
(ii) 3HCIO2 → 2HCIO3 + HCI
(iii) AgCl2 + Ag → 2AgCl
6. (c) Write oxidation half reaction and reduction half reaction and calculate EMF of the following cell at 298 K:
Fe(s)/Fe2+(aq)(0.001 M)//H+(aq)(1 M)/H2(g)(l
atm), Pt.
Standard reduction potentials of Fe2+/Fe= -0.44 V and H+ /
1/2 H2 = 0.0 V.
7. (a) Explain band theory with an example. Using the band theory
distinguish between conductors, semiconductors and insulators.
7. (b) Give the synthesis of the mixed metal carbonyl nitrosyls from the
following compounds :
(i) C5H5Mo(CO)3H and
(ii) CrCl3 and Na+ C5H5-
7. (c) Explain Structure and Bonding of ferrocene.
SECTION ‘E
(Attempt any one question)
8. (a) Briefly discuss the 'shell model of nucleus and hence explain
the concept of ‘magic numbers' for the stability of nucleus.
8. (b) Explain the concept of Nuclear Binding Energy and hence the
binding energy curve. How does the curve give information about nuclear fission
and nuclear fusion as energy source.
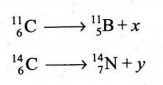
8. (c) What are x and y in the following nuclear reactions ? Justify
their formation.
9. (a) What happens, when the following salts are strongly heated ? Give balanced equations.
(i) Li2CO3
(ii) LiNO3
(iii) MgCO3
(iv) Mg(NO3)2
(v) NaNO3
(vi) Na2CO3
From these reactions, can you suggest (with reasons) a relationship between any two of these metal ions ? Suggest one more property in favour of this relationship.
9.(b) Write the formula and draw the structure of the compound which
is formed when Be(OH)2 is evaporated with acetic acid.
9.(c) Calculate the effective nuclear charge (Zeff) for the last electron
in sodium (Na) atom. (Atomic number of Na= 11)
SECTION ‘F
(Attempt any one question)
10. (a) State the method of preparation of diborane from BF3.
Draw its structure and discuss its bonding. What happens when diborane is
reacted with (i) water (ii) excess ammonia at low temperature.
10. (b) Give one example each of (i) orthosilicate (ii) pyrosilicate
(iii) cyclic silicate and (iv) chain silicate and draw their structures.
11. (a) Calculate the magnetic moment of Ce3+(f1) and Yb3+(f13) considering spin-orbit coupling. Comment on the difference (if any) in the ground. State terms and values of the moment, of the two ions.
11. (b) Explain why
(i) Atomic radii of Zr and Hf are virtually identical.
(ii) The most common oxidation state of Cu is +2 but that of Ag is +1.
(iii) Gold forms Au- ion under suitable conditions but Cu or Ag does not.
Click Here to Download PDF Chemistry Paper- I
<< Go Back To Main Page
Courtesy: UPSC

