(HOT) UPSC Current Affairs 2025 PDF
NEW! The Gist (NOV-2025) | E-BOOKS
UPSC Geo-Scientist and Geologist Exam Papers 2018 : Chemistry Paper - III

(Download) UPSC: Geologist Examination Papers-2018
Exam Name : UPSC Geo-Scientist and Geologist Exam 2018
Subject : Chemistry Paper - III
Year : 2018
CHEMISTRY
Paper III
Time Allowed : Three Hours
Maximum Marks : 200
QUESTION PAPER SPECIFIC INSTRUCTIONS
Please read each of the following instructions carefully before attempting questions.
There are TEN questions divided under TWO Sections.
Candidate has to attempt SIX questions in all.
Question no. 1 in Section A and Question no. 6 in Section B are compulsory. Of the remaining questions in each section candidates have to answer FOUR questions choosing TWO from each section.
The number of marks carried by a question / part is indicated against it.
Attempts of questions shall be counted in sequential order. Unless struck off, attempt of a question shall be counted even if attempted partly.
Any page or portion of the page left blank in the answer-book must be clearly struck off. Answers must be written in ENGLISH only.
Neat sketches may be drawn to illustrate answers, wherever required.
Unless otherwise mentioned, symbols and notations have their usual standard meanings. Assume suitable data, if necessary and indicate the same clearly.
SECTION A
Attempt any three questions including question no. 1 which is compulsory.
Q1. Answer all of the questions given below. Give reasons for the following observation :
(a) Phenolphthalein indicator
cannot be used in the titration of sodium carbonate with hydrochloric acid
solution.
(b) Iodimetric titrations are usually carried out in neutral solutions.
(c) A small amount of magnesium salt is added to the EDTA solution used for the
titration of calcium by using Eriochrome Black T indicator.
(d) Source modulation is used in atomic absorption spectroscopy.
(e) Zone broadening is inevitable in column chromatographic analysis.
(f) ICP method is better suited for multielemental analysis than flame atomic
absorption method.
(g) Glass pH electrode tends to indicate a pH lower than the actual pH in
strongly basic solutions.
(h) Electron capture detector is a selective detector.
Q2. Distinguish between the following :
(a) Co-precipitation and Post-precipitation
(b) Iodimetry and Iodometry
(c) Gas liquid chromatography and High performance liquid chromatography
(d) Proximate analysis and Ultimate analysis
(e) Aniline point and Cloud point
(f) Primary standard and Secondary standard in volumetric analysis
Q3.
(a) A mixture of HCl and H3PO4 is titrated with 0.1000 M
NaOH. The first end point (methyl red) occurs at 35.00 mL, and the second end
point (bromothymol blue) occurs at a total of 50.00 mL. Calculate the millimoles
of HCl and H3PO4 present in the solution.
(b) What weight of pyrite ore must be taken for analysis so that the BaSO4
precipitate weight obtained will be equal to one-half that of the percent S in
the sample ?
(c) (i) Explain why a wash liquid is an electrolyte in gravimetric analysis.
(ii) Calculate the solubility of silver chloride in 0:1 M NaNO3.
Given : Ksp = 1.0 × 10-10
fAg+ = 0.75 and fCI- = 0.76
Q4. (a) Explain the
principles of chelation titration indicators.
(b) Calculate the height equivalent to a theoretical plate, in cm, of a gas
chromatographic column of 3 ft length in a chromatographic analysis.
Given tR = 52.3 mm and WB = 9.0 mm
(c) (i) Calculate the absorbance of a 3.49 x 10-5 M solution of Fe
(SCN)2+ complex with maximum absorption at 580 nm. Given :
Molar
absorptivity of the complex is
7.00 x 103 L cm-1 mol-1.
(ii) A sample in a 1.0 cm cell is determined with a spectrometer to transmit 80% light at a certain wavelength. If the absorptivity of this substance at this wavelength is 2.0, what is the concentration of the substance ?
Q5.(a) Describe the role
of indicator electrode in potentiometry.
(b) An ore was analyzed for manganese content by converting the manganese to Mn304
and weighed. If a 1.52 g sample yields Mn304 weighing
0.126 g, what is the percent Mn203 in the sample ?
(c) 0.40 g of an alloy was analyzed for nickel by gravimetric method using
dimethyl glyoxime reagent. The weight of the nickel as glyoximate after washing
and drying comes to a constant weight of 0.285 g. Calculate the percentage of
nickel in the alloy. (M.Wt. of nickel dimethyl glyoximate = 290.93)
SECTION B
Attempt any three questions including question no. 6 which is compulsory.
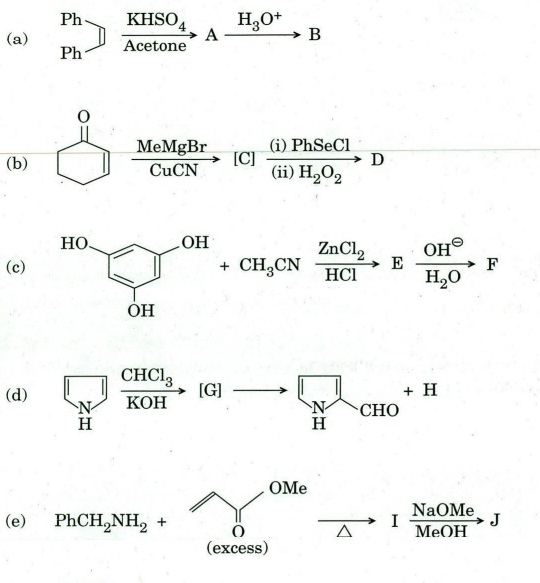
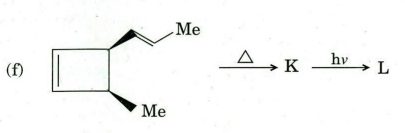
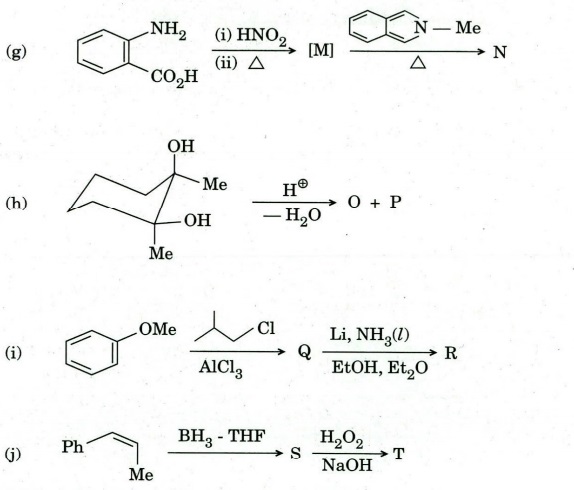
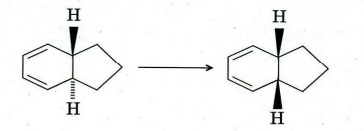
Q6. Identify compound/intermediate ‘A’ to T with appropriate stereochemistry (if any) in the following transformations :
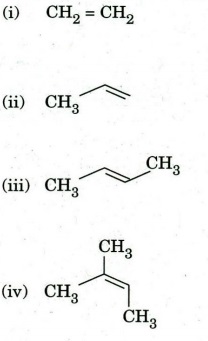
Q7. (a) Identify the most reactive alkene of the following when reacted with meta-chloroperbenzoic acid. Justify your answer.
(b) Arrange the following amines in decreasing order of their basicity. Rationalize your answer.
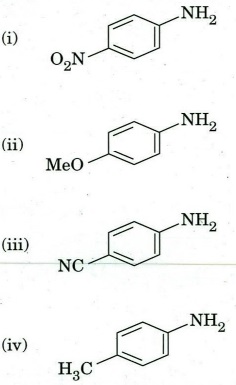
(c) Identify the product of the following transformations. Give the possible mechanism.

(d) Give a synthetic sequence for the following transformation with appropriate reagents and conditions.

(e) Suggest a suitable mechanism for the following transformation.
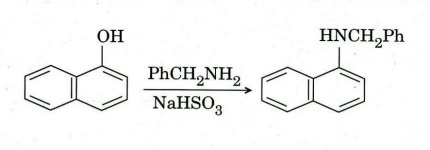
Q8. (a) Arrange the following carbocations in order of increasing stability. Justify your answer.
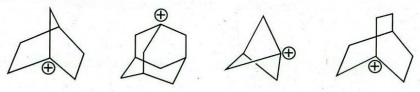
(b) Identify an anti-aromatic compound from the following. Rationalise your answer.
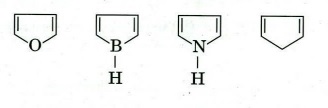
(c) Illustrate the following transformation with appropriate reagents and conditions.
(d) Arrange the following carbonyl compounds in order of increasing pKa. Rationalize the assigned order.
(CH3CO)2 CH2, CH3CHO, CH3COCH3, (CH3CO)3CH
(e) The unknown compound 'A' with molecular formula C10H160 adds 2 moles of bromine to give a tetrabromo derivative. It also forms hydrazone, reduces Fehling's solution and absorbs at 200 nm in UV. Compound ‘A’ undergoes retro-aldol reaction with boiling aqueous K2CO3 solution to give acetaldehyde and compound 'B'. The compound 'B' gives positive haloform test and yields acetone and 4-oxopentanoic acid on oxidative ozonolysis. Identify A and B and rationalize your answer.
Q9. (a) Write the
structure of the product formed when chlorotriphenylmethane is reacted with
metallic silver. Justify your answer.
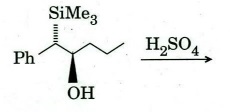
(b) Identify the product and comment on the stereochemical outcome of the
reaction.
(c) Identify the product of the following transformation. Woodward - Hoffmann
rule to explain the outcome of the reaction.

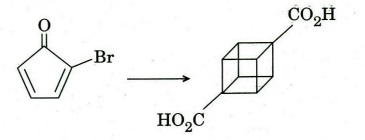
(d) Give a synthetic sequence for the following transformation with appropriate
reagents and conditions.
(e).
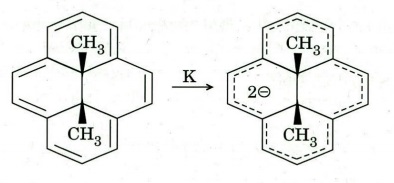
(i) Identify the reactant and the product as aromatic, anti-aromatic and
non-aromatic.
(ii) Assion the Assign the reason for appearance of methyl proton of reactant
and product at - 4.25 ppm and 21 ppm, respectively.
Q10. (a) 0-H
stretching frequency of BHT (2,6-di(tert-butyl)-4-methyl phenol) is observed at
3600 cm-1 as a sharp band, while in paracetamol (p-hydroxy
phenylacetamide), it appears as a broad band between 3300 – 3000 cm-1.
Why?
(b) The unknown compound having molecular formula C4H20
has absorption band at 1070 cm-1 in IR spectrum. In its 1H
NMR spectrum, two multiples are observed with 1:1 ratio. Identify the compound
and explain your answer.
(c) Identify the following as per given direction :
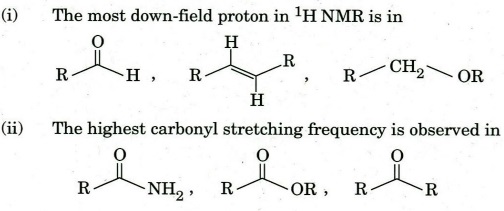
(iii). The compound exhibiting odd molecular ion peak in mass spectrum is :
BHT, DDQ, DMF
.jpg)
(d) Identify the organic compound which showed the following spectroscopic data :
UV: lmax 262 nm
IR: Significant absorption bands at 3340, 3000, 1040 cm-1
1H NMR: d 1.9 (1H, S: exchangeable with D20)
2.9 (2H, t), 3.8 (2H, t), 7.2 - 7.4 (4H, m)
13C NMR: d 39, 62, 126, 128, 129, 139
Mass : m/z 122, 92, 91
Click Here to Download PDF Chemistry Paper- III
<< Go Back To Main Page
Courtesy: UPSC

