(Download) UPSC: Geologist Examination Papers-2020 (Chemistry Paper - 2)

(Download) UPSC: Geologist Examination Papers-2020
(Chemistry Paper-2)
Exam Name : UPSC Geo-Scientist and Geologist Main Exam
Subject : UPSC Geo-Scientist and Geologist Main Exam Papers 2020 : Chemistry Paper- 2
Year : 2020
Chemistry Paper - 2
SECTION A
Q1. Answer all of the following questions: 5x16=80
(a) At the critical point on P-V isothermal plot at critical temperature (T.)

(c) Prove that greater the diffraction angle, greater is the accuracy in determining the lattice parameters.
(d) Explain how thermodynamics relates non-expansion work to a change in composition of a system.
(e) Discuss van't Hoff plots for endothermic and exothermic reactions.
(f) Calculate ionic strength I, mean ionic activity coefficient ry, and mean activity af, for a 0.0250 m solution of K2SO4 at 298 K. Assume complete ionisation.
(g) The rates of a reaction starting with initial concentration 2 x 10-3 mol L-1 and 1 x 10-3 mol L-1 are equal to 2.40 x 10-4 mol L-15-1 and 0.6 x 10-4 mol L-1 s-1, respectively. Calculate the order of the reaction with respect to the reactant and the rate constant.
(h) The activation energy of a reaction is 75.2 kJ mol-1 in the absence of a catalyst and 50.14 kJ mol-1 in the presence of a catalyst. How many times does the rate of reaction increase in the presence of a catalyst if the reaction proceeds at 25°C ?
(i) Are chemical potential and electrochemical potential of Zn2+ (aq) same? If not, write the expression for them.
(j) By potentiometer, we measure equilibrium or zero-current potential (E) of the galvanic cell. We know at equilibrium AGP,T = 0, but we write - AG = nFE, where E > 0. How will you explain it ?
(k) Describe under what condition/s an operator is Hermitian. Prove that Hermitian operators have real eigenvalues.

(m) RigidH, molecule is rotational spectrum inactive but rotational Raman spectrum active. Explain with reasons.
(n) Does ‘einstein' depend on wavelength of electromagnetic radiation ? If yes, how ? Give your answer with required expression.
(o) For the reaction : UO22+ (aq.) + (COOH)2 (aq.) → Uo22+ (aq.) + CO (g) + CO2(g) + H2O (l)
(i) What does the above reaction indicate ?
(ii) What is the role of Uo2+ here ?
Give reasons in support of your answer.
(p) Photodecomposition of HI
(g) follows the following mechanism :
HI + hv → H+I H + HI → H2 +
I + I +12
Find its quantum yield, o.
SECTION B
Attempt any six questions : 10x6=60
Q2. Gases A, B, C and D are van der Waals gases. Its van der Waals constants ('a' and 'b') values in SI units) are :
A B C D
'a' 0.6 0.6 0.2 0.005
'103 b' 0.025 0.15 0.10 0.02
(i) Which gas has highest critical temperature ?
(ii) Which gas molecule has highest molecular volume ?

(iv) Which gas is most nearly ideal behaviour at 273 K and 1.0 atm ? Explain with reason.
Q3. Potassium chloride (rock salt structure) crystal has a density of 1.98 g cm-3. The first order (200) reflections were observed at 6.5° when X-rays of 70.8 pm (Mo source) were used. Calculate the number of KCl molecules in a unit cell.
Q4. BaSO4 is not very soluble in water.
(i) Calculate the solubility product equilibrium constant, KSP for BaSO4 from the following molar free energies of formation, expressed as AFGoRT at 298 K.
BaSO4(s) Ba2+ (aq) SO2-4 (aq)
4fGo/RT - 549.53 – 226-21 - 300-34
(ii) Experimentally, 0.246 mg of BaSO4 (molar mass of BaSO4 = 233.38 g mol-1) is dissolved in 0.1 L of water at 298 KSP. What K does this fact predict, assuming that all activity coefficients are equal to 1?
(iii) Why is there difference in the values of Ksp as calculated in (i) and (ii)?
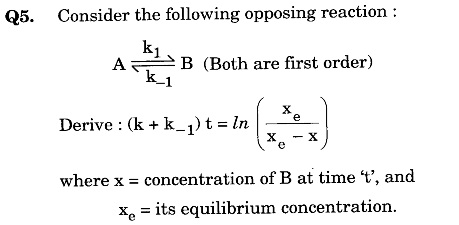
Q6. (a) Discuss the variation of molar conductivity of an aqueous solution of surfactant with the increase in concentration of surfactant solution.
(b) Why does critical micelle concentration of sodium dodecyl sulphate decrease as the concentration of added sodium chloride salt increases ? Explain this effect.

Q8. Evaluate the expectation values of p and p2 for a particle confined in the region - a/2 < x < +a/2 of a one-dimensional box.
Q9. The energy equivalent wave numbers, obtained from vibrational spectra of HD, D2, HCl and DCI in their ground level are 3627, 2990, 2885 and 1990 cm-1 respectively. Calculate the energy change (in J mol-1) of the following reaction in the ground level.
HD + DCI = D2 + HCl
Q10. (a) Fluorescence and phosphorescence are photophysical phenomena, not photochemical ones. Explain with reason.
(b) Glowing of fireflies is a result of combination of chemical and physical processes. Explain with reasons.
SECTION C
Attempt any three questions : 20x3=60
Q11. (a) Express van der Waals equation in virial form (in terms of molar volume, Vm). From this, derive the expression of Boyle temperature (TB).
(b) Show that the average position of a particle confined to the 3-dimensional box of length (a,b,c) is ( a, b, c ) is
2 2 2
Q12. The dissociation vapour pressure of NH4C1 (s) at 427°C is 608 kPa but at 459°C it has risen to 1115 kPa. Calculate (i) equilibrium constant, (ii) the standard reaction free energy, (iii) the standard enthalpy, and (iv) the standard entropy of dissociation, all at 427°C. Assume that the vapours behave as a perfect gas and that AH° and ASo are independent of temperature in the range given.
Q13. (a) For the reaction : A+B →P; derive the expression of rate constant (k) where reaction is first order with respect to each A and B and initial concentrations of A and B are ‘a’ and b' respectively.
(b) Calculate the rate constant of above reaction, when 'a' = 0.075 mol L-1; b' = 0.050 mol L-1 and concentration of B = 0.020 mol L-1 after 1 h.

Q15. (a) Define rotational constant of a molecule. How will its value change with the equilibrium bond length and reduced mass ?
(b) Bending vibration of linear carbon dioxide molecule is IR active but Raman inactive though both electric dipole moment and electrical polarisability changes with vibration. Explain with reason.
(c) Dimerisation of anthracene [A] in benzene happens by absorption of electromagnetic radiation. The concentration of its dimer at equilibrium, [A2]ep is independent of initial monomer concentration, [A], at higher concentration region. Explain with reason.

