NDA/NA Exam Solved Papers - 2012- I - Paper I: General Ability Test (Part-B)
NDA/NA Exam Solved Papers - 2012- I - Paper I: General Ability Test (Part-B)
Comprehension
Directions (Qs. 41 to 50) : Read the following three passages and answer the items that follow each passage.
Passage-I
Even in the most primitive societies, the great majority of people satisfy a large part of their material needs by exchanging goods and services. Very few people indeed can make for themselves everything they need all their food, their clothes, their housing, their tools. Ever since men started living in communities, they have been satisfying their needs by means of specialization and exchange; increasingly each individual has concentrated on what he can do best, and has produced more of the special goods or services in which he has concentrated, than he can consume himself. The surplus he has exchanged with other members of the community, acquiring, in exchange the things he needs that others have produced.
41. According to the passage, the great majority of people can satisfy their needs today by
(a) providing things for themselves
(b) exchanging goods and services
(c) concentrating on what they can do best
(d) individual specialization
42. Exchange of goods becomes possible only when
(a) there is no specialization
(b) goods are produced in surplus
(c) primitive societies become modern
(d) individuals make things for themselves
43. Specialization and exchange began when men started
(a) big industries
(b) concentrating on their work
(c) producing things for individual use
(d) living in communities
44. Exchange of goods and services becomes necessary because
(a) man is a social being
(b) reciprocity is the law of life
(c) trade and commerce are means of progress
(d) we cannot produce everything we need ourselves
Passage-II
What interests many people is the possibility of finding an Earth-like
planet, and many science fiction stories have been woven around the possibility
of there existing a planet somewhere in the universe which is an exact replica
of the Earth. There are too many variable quantities for this to be a
possibility worth considering. What is possible, if planetary systems are common
as they seem to be, is the existence of planets where the conditions are similar
to conditions on the Earth and to which our form of life could rapidly adapt. If
life had gained a foothold on such a planet, it is possible that life closely
paralleling our own planet could have developed.
What sort of conditions are necessary for life as we know it to develop? First
of all of course a suitable planetary body essential. Given this, then two vital
conditions must be satisfied. The temperature must be neither too hot nor too
cold, since intense heat breaks down organic molecules and severe cold prevents
activity from going on. Too much short-wave radiation also upsets living
organisms. The other prerequisite is a suitable atmosphere sufficiently dense to
give protection from radiation and meteorites and containing oxygen and water
vapour in reasonable quantities.
45. This passage suggests that there
(a) cannot be another planet like the Earth
(b) are other planets like the Earth mentioned only in stories
(c) may be other planets like the Earth in this universe
(d) is a planet which is exactly like the Earth
46. The hypothesis about the possibility of planets parallel to the Earth gets its strength from the fact that
(a) the scientists have discovered them
(b) books have been written about them
(c) the planetary system exists
(d) many people have shown interest in it
47. The statement, “If life had gained a foothold on such a planet” means that
(a) if there is life on the planet, it would be like ours
(b) if we go there, we can develop it like this Earth
(c) even if we try, we cannot go and live there
(d) it is impossible for life to develop there
Passage-III
“The doctor’s coming in a minute, Inspector”, said Miss Smith.
“Yes, thank you for phoning, Miss Smith. It was very kind of you ...... the
lady’s name is Mrs. West, you say,......”
“Yes, that’s right.”
“And what about Mr. West?”
“Doctor West, Inspector.”
“Oh, I see Well, Doctor West; then. Do you know where he is?”
“Not exactly. Inspector. He never told Mrs. West where he was going. You see,
they hated each other.”
“What do you mean?”
“Well. Doctor West thought that Mrs. West was in love with another mail, and
everyone knows Doctor West went to see another woman.”
48. The conversation appears to be taking place
(a) in a street where an accident has just occurred
(b) in a hotel where Mrs. West suddenly became ill
(c) in Mrs. West’s house where the police are enquiring into lady’s murder
(d) in Mrs. West’s house where a theft has taken place the night before
49. The questions the Inspector asks are
(a) inquisitive
(b) foolish
(c) disturbing
(d) searching
50. “You see, they hated each other.” “What do you mean?”
The Inspector seems
(a) to know Doctor West’s secret
(b) surprised to get the information
(c) not to have understood Miss Smith
(d) not impressed by Miss Smith’s information
Part B : General Knowledge
Directions (Qs. 51 to 59) : The following nine (09) items consists of two statements, Statement I and Statement II. You are to’ examine these two statements carefully and select the answers to these items using the codes given below:
Code:
A. Both the statements are individually true and Statement II is the correct
explanation of Statement I
B. Both the statements are individually true but Statement II is not the correct
explanation of Statement I
C. Statement I is true but Statement II is false
D. Statement I is false but Statement II is true
51. Statement I. In the year 1946, the Council of the Muslim League
accepted the Cabinet Mission Plan.
Statement II. The Muslim League proposed to
join the Interim Government.
52. Statement I. The blue colour of copper sulphate crystal disappears
when it is heated strongly.
Statement II. Due to heating, water of
crystalization of crystal is lost.
53. Statement I. After cutting an apple or a banana, the colour of the
cut surface becomes brown.
Statement II. Polyphenolic compounds present
in fruits get oxidized in air and show colour.
54. Statement I. At high temperature. hyd- rogen can reduce PbO to
elemental lead.
Statement II. Hydrogen has great affinity
to oxygen.
55. Statement I. Conversion of blue copper sulphate to black cupric
oxide on heating is a physical change.
Statement II. A change in which chemical
composition does not change is called physical change.
56. Statement I. Water is a high boiling point liquid.
Statement II. Hydrogen bonding in water is
responsible for high boiling point of water.
57. Statement I. Convex mirror is used as a driver mirror.
Statement II. Images formed by convex mirror
are diminished in size.
58. Statement I. A thermos flask is made of double-walled glass
bottles.
Statement II. Metals are good conductors
while gas and air are poor conductors of heat.
59. Statement I. Bats can catch their prey in the darkness of night.
Statement II. Bats can produce and also
detect ultrasound.
60. The Indian Standard Time (IST) is based on
(a) 90° E meridian
(b) 82Emeridian
(c) 75° E meridian
(d) 0° meridian
61. Collision-coalescence process of precipitation is applicable to
(a) those clouds which do not extend beyond the freezing level
(b) those clouds which extend beyond the freezing level
(c) all types of clouds
(d) cumulonimbus clouds
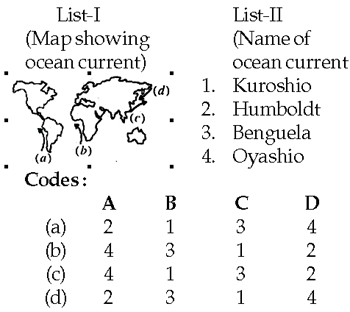
62. Match List-I with List-II and select the correct answer using the codes given below the Lists:\
63. Which one among the following nutrients is a structural component of the cell wall of plants?
(a) Manganese
(b) Potassium
(c) Phosphorus
(d) Calcium
64. Balanced diet. should have approximately
(a) 1/5 protein, 3/5 fat and 1/5 carbohydrate
(b) 3/5 protein, 1/5 fat and 1/5 carbohydrate
(c) 1/5 protein, 1/5 fat and 3/5 carbohydrate
(d) 1/2 protein, 1/4 fat and 1/4 carbohydrate
65. Which one among the following statements is correct?
(a) All arteries Carry oxygenated blood
(b) All veins carry oxygenated blood
(c) Except the pulmonary artery, all other arteries carry oxygenated blood
(d) Except the pulmonary vein. all other veins carry oxygenated blood
66. What are the cold-blooded animals?
(a) Animals with blood without hemoglobin
(b) Animals who are not ferocious
(c) Animals whose body temperature remains constant
(d) Animals whose body temperature varies according to the temperature of
atmosphere
67. Sickle-cell anaemia is a disease caused due to the abnormality in
(a) white blood cells
(b) red blood cells
(c) thrombocytes
(d) blood plasma composition
68. Carbohydrates are stored in plants and animals in the form of
(a) cellulose and glucose respectively
(b) starch and glycogen respectively
(c) starch and glucose respectively
(d) cellulose and glycogen respectively
69. Which of the following statements regarding oxidation and reduction are correct?
1. In oxidation, loss of electron takes place whereas in reduction, gain of
electron takes place.
2. In oxidation, gain of electron takes place whereas in reduction, loss of
electron takes place.
3. Oxidizing agent decreases the oxidation number but reducing agent increases
the oxidation number.
4. Oxidizing agent increases the oxidation number but reducing agent reduces the
oxidation number.
Select the correct answer using the code given below:
Code :
(a) 1 and 3
(b) 2 and 4
(c) 2 and 3
(d) 1 and 4
70. Which one among the following is correct regarding 20Ne, 23Na+, 19F–, and 24Mg2+?
(a) They are isomers of each other
(b) They are isotopes of each other
(c) They are isoelectronic with each other
(d) All of the above
71. Which of the following pairs is/are correctly matched?
1. Isotopes : Atoms with same atomic number but different atomic mass
2. Isobars : Atoms with same number of neutrons but different atomic number
3. Isotones : Atoms with same mass number but different atomic number
Select the correct answer using the code given below:
Code :
(a) 1, 2 and 3
(b) 1 only
(c) 1 and 2 only
(d) 2 only
72. Sometimes, indigestion is caused by the secretion of too much hydrochloric acid in the stomach. To ease the pain caused, a tablet can be taken that reacts to reduce the amount of acid present. Which one among the following would be inappropriate for a manufacturer to include as a major reactant in the tablet?
(a) CaCO3
(b) MgCO3
(c) NaOH
(d) Mg(OH)2
73. Muhammad bin Tughlaq’s experiment of introducing token currency could not succeed on account of
(a) rejection of token coins by foreign merchants
(b) shortage of copper for minting token coins
(c) large-scale minting of spurious coins
(d) poor quality of token currency
74. The Parliament can legislate on the subjects ill the State List if the
(a) President issues an order authorizing it to do so
(b) Supreme Court gives authority it the Parliament in this regard
(c) Rajya Sabha passes a resolution by two-thirds of its members present and
voting, declaring it expedient to legislate on a State matter in the national
interest
(d) Prime Minister issues a special order
75. The writ of certiorari is issued by a superior court to
(a) an inferior court to stop further proceedings in a particular case
(b) an inferior court to transfer the record of proceedings in a case for review
(c) an officer to show his/her right to hold a particular office
(d) a public authority to produce a person detained by it before the court
within 24 hours
76. Schemes run under the National Rural Employment Guarantee Act are sponsored
(a) by the Central Government alone
(b) partly by the Central Government and partly by the State Government
(c) by Centre, State and Panchayat bodies together
(d) on public-private partnership basis
77. The World Bank was created immediately after the Second World War. Its activities are focused on the developing countries. Which among the following are the activities or the Bank’?
1. Human development
2. Agriculture and rural development
3. Environmental protection and governance
4. Loans and grants to the member countries
Select the correct answer using the code given below:
Code :
(a) 1, 2 and 3 only
(b) 3 and 4 only
(c) 2 and 4 only
(d) 1, 2, 3 and 4
78. A refracting telescope consists of
(a) one concave mirror and one convex lens
(b) two convex lenses of equal focal length
(c) two concave mirrors of different focal lengths
(d) two convex lenses of unequal focal lengths
79. The nucleus of a singly ionized carbon atom contains
(a) 6 protons and 6 neutrons
(b) 5 protons and 6 nutrons
(c) 6 protons, 6 neutrons and 6 electrons
(d) 12 protons, 6 neutrons and 6 electrons
80. Two similarly charged bodies are kept 5 cm apart in air. If the second body is shifted away from the first by another 5 cm, their force of repulsion will be
(a) doubled
(b) halved
(c) quadrupled
(d) reduced to one-fourth
ANSWER
41. (b) 42. (b) 43. (d) 44. (d) 45. (c) 46. (c) 47. (a) 48. (a) 49. (a) 50.
(c)
51. (a) 52. (a) 53. (a) 54. (a) 55. (d) 56. (a) 57. (a) 58. (a) 59. (a) 60. (b)
61. (b) 62. (d) 63. (d) 64. (c) 65. (c) 66. (d) 67. (b) 68. (b) 69. (d) 70. (c)
71. (b) 72. (c) 73. (c) 74. (c) 75. (b) 76. (b) 77. (d) 78. (d) 79. (a) 80. (d)
Study Material for UPSC NDA/NA Examination
Books for UPSC NDA/NA Examination
Courtesy: UPSC


